Knowing the age composition within populations of fish helps to ensure the community is healthy and managed correctly.
Fish are a diverse group in which species have very variable life spans. They include the shortest and longest living vertebrates, from the adorned dwarf goby, Eviota sigillata, that has a full life cycle in just eight weeks, to the Greenland shark, Somniosus microcephalus, which is estimated to live 300 to 500 years! (1). Fish do not stop growing throughout their lifespans, although growth slows with age and the amount of growth varies between species. As with humans, the size of fish does not correlate directly with age, but measuring the size of fish can provide an estimate for age within species populations to give an easy scale of when a species has reached maturity and is likely to reproduce. This helps us to manage the population to ensure continued sustainable growth.
The relationship between age and size is determined through examining multiple individuals of a species where the age and length can be accurately determined and compared. This growth rate has been determined for many species, and recorded on the FishBase database (2). A database on the natural mortality rates and associated life history parameters is in development by Then et al at Virginia Institute of Marine Science (3,4). Once the relationship between age and size in a species has been determined, size can be used to estimate the age of fish, using the von Bertalanffy growth equation (5,6). This equation is also used to estimate when a percentage of the population reaches maturity. The natural mortality rate can be calculated using the Pauly (1980) equation (7, 8). Though there are other equations used for the same purpose, these are the most widely used equations for fisheries studies (9). These statistics can then be used to set minimum landing sizes, so that fishing is managed as sustainably as possible.
Aging bony fish
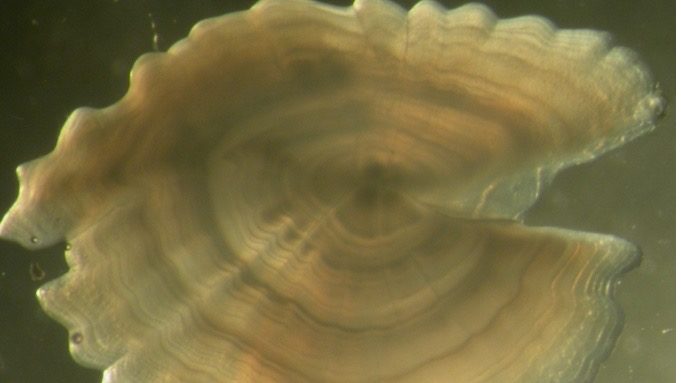
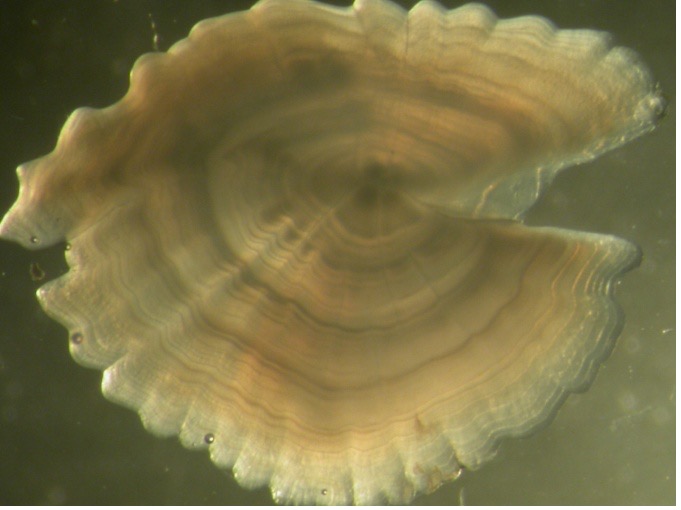
Osteichthyes, or bony fish, account for 96% of all fish species. The aging of these fish is done using their bony structures, such as the spinal vertebrae, the fin spines, opercular bones (bony plate covering the fish’s gills), otoliths (“earstones”), and scales. These structures are made up of thin layers called circuli, which are built up over the individual’s life span, allowing age to be estimated by counting the number of these growth rings, similar to aging a tree.
The least invasive way to age a fish is to assess the scales, as these can be detached from living specimens. However, scales can be shed and regrown, which could cause inaccurate age estimates and the rings are hard to distinguish in certain species and in older fish (10). Internal structures are therefore the most reliable, especially the otoliths, which are usually the easiest to read and give an accurate representation of the life history of the fish, as they are the first bony structures that develop. However, otolith extraction does require the death of the fish.
Otoliths form part of the inner ear of fish. As fish grow, otoliths increase in size through the deposition of layers. Numerous seasonal factors, such as water temperature and food availability, affect growth rates in fish and they will grow rapidly during the warm season, with growth slowing or stopping during winter. This changing pattern of growth is reflected in the ring structure of otoliths. During rapid growth, a white, opaque band is deposited, while during periods of slow growth, the band is dark and translucent. In temperate waters, usually one white and one dark band are laid down each year. Each pair of bands is referred to as an annular mark and by counting the number of annuli outside the nucleus of the otolith it is possible to age the fish.
Otoliths come in different shapes and sizes depending on the species. In some, such as plaice and mackerel, the otoliths are thin, and the bands are easy to distinguish. Other species, such as whiting and cod, require the otolith to be sectioned before the ring pattern can be seen.

Aging cartilaginous fish
Some scaleless fish have skeletons made up of cartilage instead of bone. This lack of hard structures makes determining the age of these species more difficult and many aging techniques are still being developed and refined.
One way a cartilaginous fish can be aged is through the use of tagging. Individuals are measured then tagged, so that when they are recaptured, they can be measured again, allowing for an average growth to be calculated and compared over time. Tags can be physical or chemical or, in some species, identifiable markings of individuals can be used. This technique is used alongside the examination of deceased individuals to determine the relationship between age and size in a population. In deceased individuals, the cartilaginous fine spines or vertebrae from the backbone of some species can be used, counting the rings much like in the bony structures of other species, although they need to be sectioned in a specific way and it is not currently as well refined in technique (11, 12).
Cartilaginous fish can also be aged via radiocarbon dating of the eye lens nucleus. In vertebrates, the eye lens nucleus is composed of metabolically inert proteins. The centre is formed during prenatal development and the inert proteins are retained throughout the lifetime of the individual. This unique feature of the eye lens has been exploited for difficult-to-age vertebrates such as Greenland sharks (1). Radiocarbon dating results and the size and gender of the individuals tested can be used to create growth curves for predicting the age of other individuals of the species from their size.
The importance of age
Age data can tell us how long fish live, their rate of growth and their age at critical periods during their lives. If the age range in a population is broad, then the stock would appear to be in good shape. If there are no young fish, then recruitment (spawning) may not be occurring and the stock will collapse. Alternatively, if there are no old fish, it is a sign the stock may be being overfished (12, 13). Additionally, age information can be used to judge the effects of management practices such as size limits. Species need protection so that females have a chance to grow to maturity and produce offspring to continue the population, particularly species that grow very slowly, such as sturgeons and sharks.
- Nielsen, J., Hedeholm, R.B., Heinemeier, J., Bushnell, P.G., Christiansen, J.S., Olsen, J., Ramsey, C.B., Brill, R.W., Simon, M., Steffensen, K.F. and Steffensen, J.F., 2016. Eye lens radiocarbon reveals centuries of longevity in the Greenland shark (Somniosus microcephalus). Science, 353(6300), pp.702-704.
- Froese, R. and D. Pauly. Editors. 2021. FishBase. World Wide Web electronic publication. www.fishbase.org, version (06/2021).
- Then, A.Y., and J.M. Hoenig. 2015. Database on Natural Mortality Rates and Associated Life History Parameters, version 1.X. Available at: http://bit.ly/vims_mort.
- Then, A. Y., J. M. Hoenig, N. G. Hall, and D. A. Hewitt. 2015. Evaluating the predictive performance of empirical estimators of natural mortality rate using information on over 200 fish species. ICES Journal of Marine Science 72:82–92.
- Froese, R., 2006. Cube law, condition factor and weight–length relationships: history, meta‐analysis and recommendations. Journal of applied ichthyology, 22(4), pp.241-253.
- King, M. (2007). Fisheries Biology, Assessment and Management. 2nd 366 Edition. Blackwell Publishing, Oxford, UK, 382 pp. ISBN 9781405158312
- Pauly, D., 1980. A new methodology for rapidly acquiring basic information on tropical fish stocks: growth, mortality and stock-recruitment relationships. In Stock assessment for tropical small-scale fisheries. Proc Int Workshop Univ Rhode Island, Narragansett(pp. 154-172).
- Pauly, D., 1980. On the interrelationships between natural mortality, growth parameters, and mean environmental temperature in 175 fish stocks. ICES journal of Marine Science, 39(2), pp.175-192.
- Henderson, P., Seaby, R. and Somes, R., 2006. Growth II. Pisces Conservation Ltd, Lymington, England.
- Khan, M.A. and Khan, S., 2009. Comparison of age estimates from scale, opercular bone, otolith, vertebrae and dorsal fin ray in Labeo rohita (Hamilton), Catla catla (Hamilton) and Channa marulius (Hamilton). Fisheries Research, 100(3), pp.255-259.
- Campana, S.E., 2014. Age determination of elasmobranchs, with special reference to Mediterranean species: a technical manual. General Fisheries Commission for the Mediterranean. Studies and Reviews, (94), p.I.
- Carbonara, P. and Follesa, M.C., 2019. Handbook on fish age determination: a Mediterranean experience. General Fisheries Commission for the Mediterranean. Studies and Reviews, (98), pp.I-179.
- Campana, S.E. and Thorrold, S.R., 2001. Otoliths, increments, and elements: keys to a comprehensive understanding of fish populations?. Canadian Journal of Fisheries and Aquatic Sciences, 58(1), pp.30-38.